SGU Episode 640
| This episode needs: transcription, time stamps, formatting, links, 'Today I Learned' list, categories, segment redirects. Please help out by contributing! |
How to Contribute |
| SGU Episode 640 |
|---|
| October 14th 2017 |
| (brief caption for the episode icon) |
| Skeptical Rogues |
| S: Steven Novella |
B: Bob Novella |
C: Cara Santa Maria |
J: Jay Novella |
E: Evan Bernstein |
| Quote of the Week |
I am not very optimistic about people’s ability to change the way they think, but I am fairly optimistic about their ability to detect the mistakes of others. |
| Links |
| Download Podcast |
| Show Notes |
| Forum Discussion |
Introduction[edit]
You're listening to the Skeptics' Guide to the Universe, your escape to reality.
Forgotten Superheroes of Science ()[edit]
- Frances Glessner Lee: Frances Glessner Lee 1878 – 1962 is known as the mother of forensic science
News Items[edit]
Debunking Works (Sort of) ()[edit]
Mindfulness Pseudoscience ()[edit]
Columbus Myths ()[edit]
Missing Matter ()[edit]
Who's That Noisy ()[edit]
- Answer to last week: Water
Questions and Emails[edit]
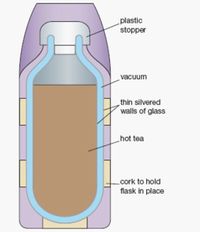
Question #1: Thermos Physics ()[edit]
Hi guys, I have a random question I'm hoping you can help me with. I have an insulated steel thermos made by Yeti. Best insulated cup I've ever used, hands down. I have no affiliation with them, FYI. If I put hot coffee in it, no warmth registers on the outside. The cup's insulating design is working as intended. The temperature delta here is roughly 80 degrees (150 degree coffee -> 70 degree ambient temp). That's pretty impressive in my book. But if I put an iced drink in, the outside of the cup becomes pretty cool pretty quickly. It still keeps the drink pretty cold, but I'm confused as to why it does not seem to be insulating as well. Here the temperature delta is only about 40 degrees (70 degree ambient temp -> 30 degree drink). So it seems to be insulating much better with a warm liquid, but I just can't make sense of this. I don't see why it would matter – energy is energy, after all, and I would think it's the temperature delta that matters more. Thoughts? My only guess is that the solid ice contacting the cup's inner wall is cooling it down more than expected? Thanks guys. Love the show btw. Keep up the good work! -Tighe Los Angeles
Science or Fiction ()[edit]
Item #1: Engineers have built a usable nano-battery using waste graphite that has sufficient energy density to run a pacemaker for 20 years. Item #2: Researchers have developed an air-breathing flow battery usable for grid storage that costs 20% as much as lithium-ion batteries with nearly as much energy density. Item #3: A new analysis finds that there are enough raw materials available to meet projected demand for lithium-ion battery production for at least 15 years.
Skeptical Quote of the Week ()[edit]
“I am not very optimistic about people’s ability to change the way they think, but I am fairly optimistic about their ability to detect the mistakes of others.” - Daniel Kahneman
S: The Skeptics' Guide to the Universe is produced by SGU Productions, dedicated to promoting science and critical thinking. For more information on this and other episodes, please visit our website at theskepticsguide.org, where you will find the show notes as well as links to our blogs, videos, online forum, and other content. You can send us feedback or questions to info@theskepticsguide.org. Also, please consider supporting the SGU by visiting the store page on our website, where you will find merchandise, premium content, and subscription information. Our listeners are what make SGU possible.
References[edit]

|